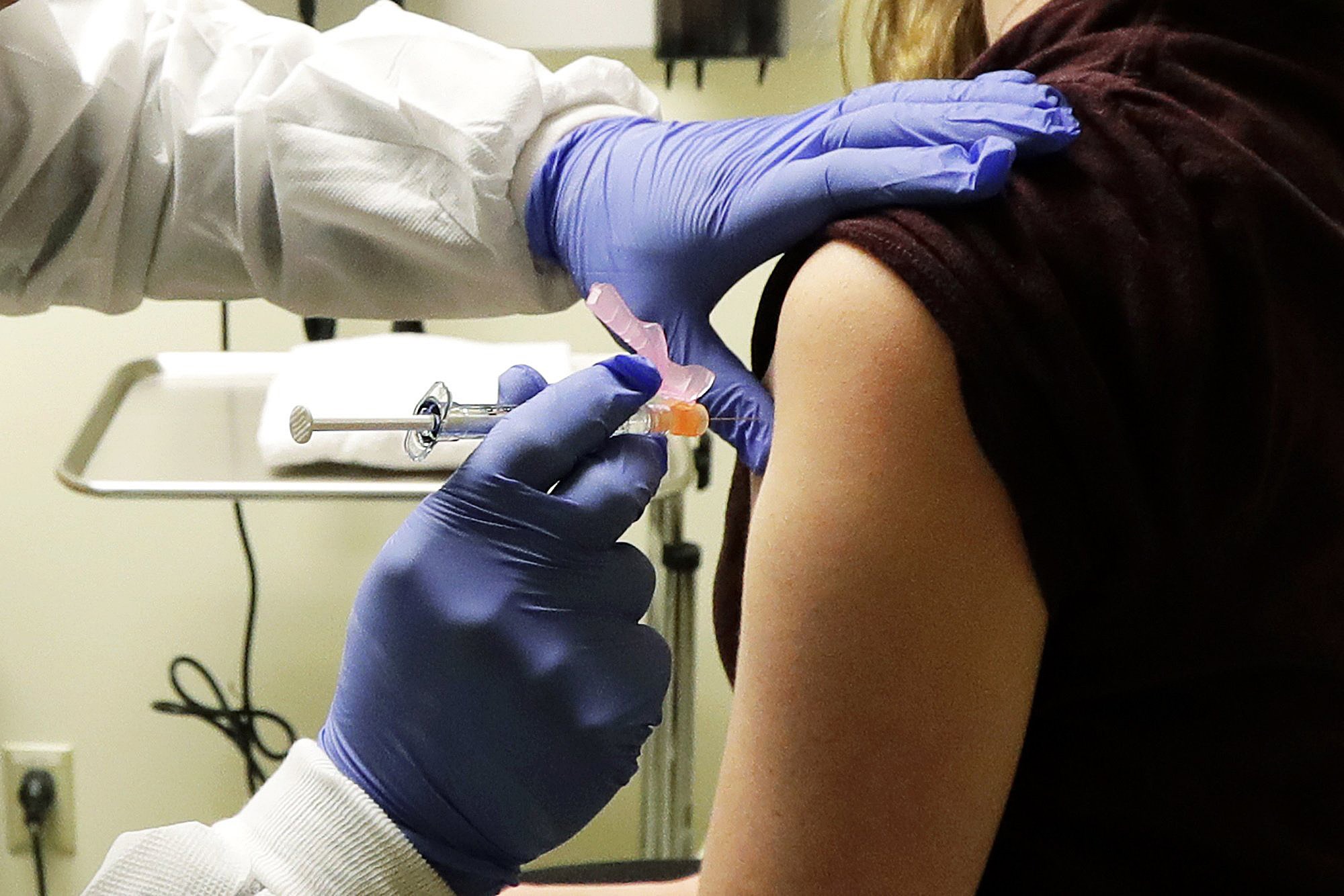
The Food and Drug Administration (FDA) on Monday granted full approval to Moderna’s COVID-19 vaccine, giving an additional vote of confidence in its safety and effectiveness.
The full approval for people ages 18 and older was based on follow-up data showing “high efficacy and favorable safety approximately six months after the second dose.”
The vaccine had already been available since December 2020 under an emergency use authorization, but full approval provides an extra emphasis.
Acting FDA Commissioner Janet Woodcock said she hoped the move gives some people additional confidence in the vaccine.
Read more at thehill.com